
Full text loading...
Three thermophilic, aerobic, hydrogen- and sulfur-oxidizing bacteria were isolated from an Icelandic hot spring near the town of Hveragerdi and share >99 % 16S rRNA gene sequence similarity. One of these isolates, designated strain I6628T, was selected for further characterization. Strain I6628T is a motile rod, 1.5–2.5 μm long and about 0.5 μm wide. Growth occurred between 40 and 73 °C (optimally at 68 °C), at pH 5.3–7.8 (optimally at pH 6.6) and at NaCl concentrations between 0 and 0.5 % (w/v). Strain I6628T grew with H2, S0 or 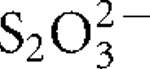 as an electron donor with O2 (up to 25 %, v/v; optimally at 4–9 %) as the sole electron acceptor. CO2 and succinate were utilized as carbon sources but no organic compounds, including succinate, could be used as an energy source. The G+C content of the genomic DNA was determined to be 28.1 mol%. Phylogenetic analysis of the 16S rRNA gene sequence indicated that strain I6628T is a member of the genus Sulfurihydrogenibium, the closest cultivated relative being the recently described strain Sulfurihydrogenibium rodmanii UZ3-5T (98.2 % sequence similarity). On the basis of the physiology and phylogeny of this organism, strain I6628T represents a novel species of the genus Sulfurihydrogenibium, for which the name Sulfurihydrogenibium kristjanssonii sp. nov. is proposed. The type strain is I6628T (=DSM 19534T =OCM 901T =ATCC BAA-1535T).
as an electron donor with O2 (up to 25 %, v/v; optimally at 4–9 %) as the sole electron acceptor. CO2 and succinate were utilized as carbon sources but no organic compounds, including succinate, could be used as an energy source. The G+C content of the genomic DNA was determined to be 28.1 mol%. Phylogenetic analysis of the 16S rRNA gene sequence indicated that strain I6628T is a member of the genus Sulfurihydrogenibium, the closest cultivated relative being the recently described strain Sulfurihydrogenibium rodmanii UZ3-5T (98.2 % sequence similarity). On the basis of the physiology and phylogeny of this organism, strain I6628T represents a novel species of the genus Sulfurihydrogenibium, for which the name Sulfurihydrogenibium kristjanssonii sp. nov. is proposed. The type strain is I6628T (=DSM 19534T =OCM 901T =ATCC BAA-1535T).

Article metrics loading...

Full text loading...
References


Data & Media loading...
Supplements
